Oncomedicine 2017; 2:132-137. doi:10.7150/oncm.20393 This volume Cite
Review
BRCA 1/2 Tumors and Gene Expression Therapy for Breast Cancer Development and Metastasis
1. Department of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 38117. USA;
2. Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Received 2017-4-3; Accepted 2017-5-3; Published 2017-6-29
Abstract
The rise of bioinformatics has allowed for alternate therapies in treating disease. Thus, researchers have discovered relationships between the breast cancer gene (BRCA) family and the cancer itself. Recent investigations by the National Cancer Institute have shown that almost 55% of women with defective BRCA genes develop breast cancer by age seventy. These genes play an important role in tumor suppression; their mutations can increase the probability of developing metastatic cancer. Hence, scientists have begun studying BRCA-targeted gene therapy as an effective treatment for breast cancer.
This paper investigates the mechanisms of BRCA1 and BRCA2 gene expression therapy in patients with breast cancer. By approaching the disease and its biochemical implications, it seeks to review current treatment options and genetic studies. We discuss the critical role of the BRCA gene family in signaling pathways (including angiogenesis) and evaluate the impacts of the loss-of-function mutations associated with the aforementioned disease. With this, we make suggestions for future studies and optimal treatment plans.
Keywords: BRCA1, BRCA2, gene expression therapy, breast cancer
Introduction
Breast cancer affects one in eight women over the course of her lifetime. Its etiology is unknown; however, strong correlations are noted between genetic factors and the incidence of the disease. Currently, scientists have investigated ties between estrogen levels and disposition to breast cancer. They have identified a direct relationship between mutations in the BRCA gene family and the probability of malignancy. [2]
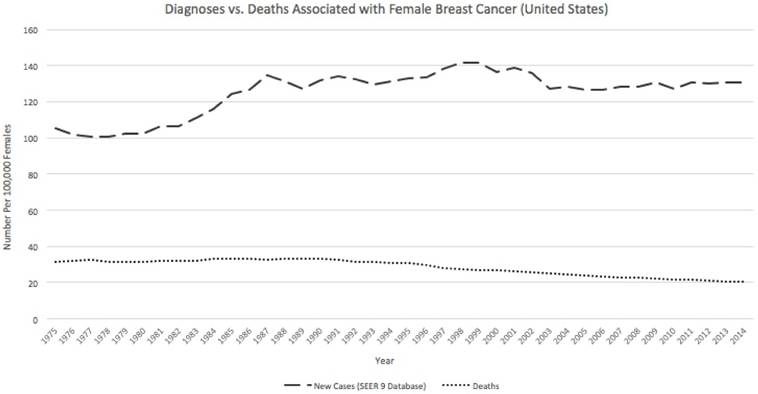
In addition, biologists have found a constant death rate among breast cancer patients. The rate of diagnosis also appears to remain constant over time (Figure 1). [3] This suggests that modern advances in treatment have remained largely ineffective in reducing the malignancy of the cancer.
Further research reveals that the cancer is most often diagnosed in women ages forty-five to seventy-four. [4] This supports a tie between hormonal change and disposition to breast cancer, as menopause coincides with much of this age interval.
With this in mind, cancer biologists have proposed gene expression therapy to reduce death rates among middle-aged women. Current targets of interest include progesterone and estrogen receptors and the BRCA1 and BRCA2 regulatory genes. [1,5] These genes and their protein products were first identified in 1990 and 1994, respectively, by gene mapping initiatives in the U.S. and the U.K. [6,7]
The rate of diagnosis of breast cancer has remained relatively constant over time. In addition, the death rate has not decreased with the advent of new therapies. This indicates that new therapies are largely inefficient at reducing the incidence of breast cancer. Adapted from Ref. 3, http://seer.cancer.gov/statfacts/html/breast.html
Breast Cancer Development and Metastasis
Scientists have classified breast cancers using many diagnostic criteria. Touchstones include the location of tumors, the appearance of cancerous cells, and the genomic makeup of the cancer.
Breast cancer often begins in the milk ducts of an affected woman. This disease is called ductal carcinoma and can be further classified by its direction of travel. Ductal carcinoma in situ remains in the epithelial cells of the ducts, while invasive ductal carcinoma spreads to other parts of the breast. If the cancer begins in the lobular tissue, it is named lobular carcinoma. Invasive lobular carcinoma spreads across other mammary tissues and can metastasize. Bone and blood-related cancers can also occur in the breast; these sarcomas are rare and may also become invasive.
Breast cancers are rated upon microscopic analysis. Using various rubrics, scientists score a tumor from one to three based on its relative appearance to normal breast cells. Tumors rated at three are the most aggressive, while those at one are the least dangerous.
Genetically, classifications also arise from hormone-dependence. Some cancerous cells contain extracellular receptors for estrogen and progesterone; these proteins are able to capture free-floating hormones in the body. Thus, breast cancers can be estrogen receptor (ER) positive, progesterone receptor (PR) positive, or hormone receptor negative (HR). Tumors are also rated on the presence of the HER2 gene. This gene segment promotes growth of the cell culture and can lead to cancer upon gain-of-function mutations. Hence, breast cancers are either luminal A, luminal B, HER2 positive, or basal-like. Luminal A cancers are ER positive, PR positive, and HER2 negative, while luminal B tumors are ER positive, PR negative, and HER2 positive. HER2 positive breast cancers are ER and PR negative but HER2 positive. Finally, basal-like tumors are negative for all genetic traits. [5]
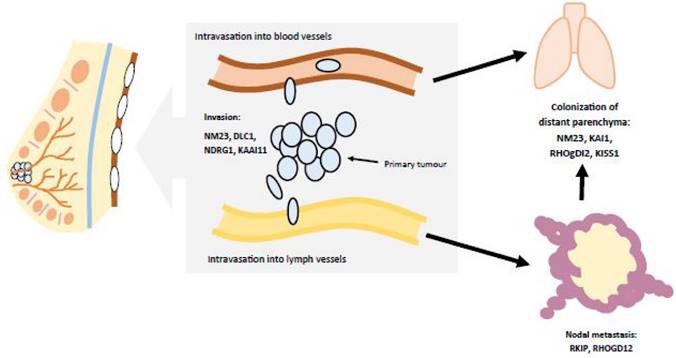
The manifestations of breast cancer are similar to many other cancers. By this, there is excess mitotic activity in breast tissue. Tumors continue to grow as mutated DNA is donated to each daughter cell. The mutation affects cellular machinery, including tumor suppressor genes (e.g., p53), cyclins/cyclin-dependent kinases, cell cycle checkpoints, etc. As the tumor develops, it then begins to spread. This metastasis is facilitated by the invasion of cancerous cells into blood vessels and lymph canals. Thus, breast cancer is able to travel in the bloodstream and affect other organ systems (Figure 2). [8]
Each cancer usually affects a set group of extraneous tissues. For breast cancer, these include a woman's bones, brain, liver, and lungs. Cancer becomes more highly rated as it metastasizes; hence, metastatic breast cancers are scored more closely to three. This suggests that a metastasized tumor presents enhanced danger to a patient's health.
Breast cancer metastasizes as the tumor spreads from the breast to other organs. This occurs as the tumor enters blood, lymph, and/or other fluids. Adapted from Ref. 8.
Metastatic cancers often create comorbidities in affected patients. Symptoms of these disorders can be used to diagnose metastasis; however, most diagnoses are made using x-rays and other specialized tests. Often, new conditions indicate cancerous growth in a specific area of the body. Increased bone fractures may indicate newfound neoplasms, while seizures and headaches can suggest metastases in the brain. [9]
These medical observations facilitate personalized treatments for patients. Most often, affected women are prescribed medicines and advised to pursue other chemotherapeutic treatments. Nonetheless, an oncologist might recommend a mastectomy to severely reduce the chance of metastasis. He or she may also recommend hormonal therapies if the cancer is hormone-dependent. These include prescription drugs that regulate estrogen activity and formation (aromatase inhibitors and selective estrogen receptor modulators, e.g.). The affected woman is further advised to exercise routinely and watch her diet. [10]
BRCA Genes 1 and 2
As research continues to develop, new studies suggest the effectiveness of targeting the BRCA gene family in gene therapy treatments. Inherited mutations in these genes leads to a significantly-increased probability of developing breast cancer in at-risk women. [1]
Advanced genomic sequencing has located the BRCA1 gene on the long arm of chromosome seventeen. This section encodes a phosphoprotein with an affinity for damaged genetic material. Hence, BRCA1 proteins combine with other tumor suppressing proteins and signal transducers to form the large BRCA1-associated genome surveillance complex (BASC). BASC binds to RNA polymerase II and histone deacetylase complexes, thus regulating gene expression at the transcriptional level. As BASC prevents the acetylation of specific histones, it leads to the downregulation of transcription. BRCA1 also repairs double-stranded DNA breaks and regulates recombination. [11]
In contrast to BRCA1, BRCA2 is found on the thirteenth chromosome. It works in conjunction with BASC, helping to repair broken DNA and regulate recombination of ligated strands. Primarily, this occurs through its binding with the ligase RAD51 recombinase. [12]
Through these regulatory processes, the BRCA genes and the BASC complex reduce the probability of tumorigenesis. Errors in BRCA proteins often stem from nonsense mutations in the transcription of the BRCA1 and BRCA2 genes. Hence, the resulting polypeptides are overly short and nonfunctional. The proteins are unable to remedy errors in the DNA; as these faults accumulate, they promote the development of a tumor. Specifically, BRCA gene mutations have been implicated in breast, ovarian, prostate, colon, and pancreatic cancers. [13]
In addition, the transcription factor HIF-1α (hypoxia inducible factor 1α) has been implicated in the aggressiveness of BRCA tumors. HIF-1α is highly conserved and upregulates angiogenesis in cells deprived of oxygen. Through redirection of hemoglobin, hypoxic cells are compensated with adequate oxygen and nutrients. [14] Oxygen, the final electron acceptor in the electron transport chain of cellular respiration, is essential to the cell, as it allows the manufacturing of adenosine triphosphate (ATP) through oxidative phosphorylation. This makes HIF-1α an essential transcription factor for cell survival.
BRCA tumors often involve the overexpression of the genes that code for the HIF-1α subunit. This provides tumors with excess nutrients and promotes its metastasis through continued angiogenesis. Hence, the overproduction of HIF-1α leads to excess dimerization of the transcription factor with the subunit HIF-1β. The united HIF-1 complex then binds with the promoter regions of its target genes, leading to angiogenesis. As new blood vessels are formed and directed towards the tumors, the cancer metastasizes at a faster rate. [15]
With overexpression of HIF-1, BRCA tumors are further supported by the angiogenic role of vascular endothelial growth factor (VEGF). This ligand is produced when HIF-1 α acts as a transcription factor, allowing the transcription and translation of the genes coding for VEGF. When VEGF is produced, angiogenesis is initiated, as the ligand binds to VEGF receptors on endothelial cells. The resulting signal transduction pathway allows for tumor proliferation through vascularization of the endothelium. [16]
New studies have supported the heavy upregulation of the VEGF pathway in familial BRCA tumors. In a recent investigation, Saponaro, et al. found that VEGF density in BRCA 1/2 tumors was significantly increased compared to BRCAX and other sporadic cancers. [17] This suggests the role of angiogenesis in promoting aggressive metastasis of the cancer.
Approaches to Gene Therapy
Targeted molecular therapies are under development for breast cancer and specific strains of BRCA tumors.
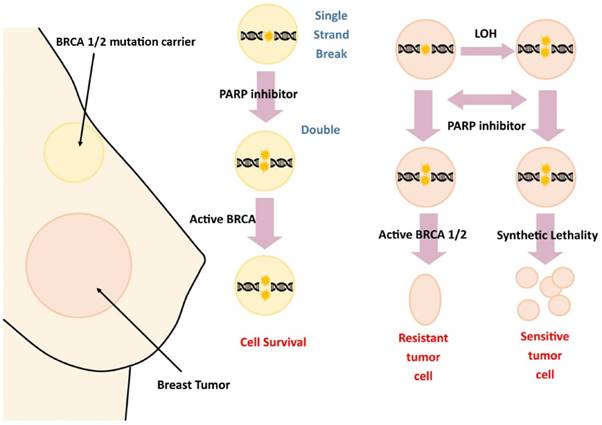
Noting the implications of damage to the BRCA gene family in breast cancer development and metastasis, cancer biologists have begun research into targeted therapies. Of recent interest are poly adenosine diphosphate ribose polymerase inhibitors (PARPi), a class of drugs targeting DNA repair enzymes in damaged cells. Specifically, these targets have shown promising results in BRCA tumors, as they prevent the catalysts from repairing DNA in metastasizing cancer cells (Figure 3). Mutations in BRCA genes lead to their inactivity; thus, individuals with BRCA tumors do not undergo recombination at the BRCA1 and BRCA2 loci. This puts affected cells at higher risk for tumorigenesis, as the cell's regulatory ability is diminished. PARPi medications specifically target cells with non-recombined chromosomes. These proteins consist of seventeen subgroups largely involved in the repair of double strand breaks; thus, PARPi therapies effectively block the ability of at-risk cells to repair DNA damage. Furthermore, PARPi is selective; therapy is applied only to cells with a defective gene. This has made the inhibitors extremely promising in pharmacological development. [18]
PARPi therapy targets the ability of BRCA 1/2 to promote repair of DNA double-stranded breaks.
Other therapies target the BRCA gene itself. Previous studies have documented the inverse relationship between expression of BRCA and the incidence of breast cancer. Thus, de-methylating, selectively-repressed sections of the gene have shown to be effective in reducing the incidence of tumors. The PA-1 tumor model has shown promising results, as BRCA viral vectors were introduced into the peritoneum. The high density of the BRCA gene was further associated with diminished tumor expression. Targeted p53 gene therapy is also promising, as literature has documented the role of introduced p53 in slowing the spread of many cancerous variants. [19]
In addition, some publications have documented the efficacy of BRCA as a biomarker in chemotherapeutic medicines. James, et al. detail the oncological applications of BRCA density testing to predict cellular response to chemotherapy drugs. While ER and HER2 act as biomarkers for hormonal treatment and immunotherapy, respectively, BRCA determines the efficacy of chemotherapy. Thus, downregulation of BRCA1 signals increased reception of cisplatin and mitomycin C, two key chemotherapeutic agents creating DNA double-stranded breaks and leading to apoptosis of cancer cells. Because BRCA tumors involve downregulation of the gene, it was noted in the human cell line HCC1937 that these drugs worked well when BRCA had no effect on DNA. In addition, downregulation of BRCA signals increased resistance to microtubule-interfering agents like vinca alkaloids and taxanes. As mRNA-specific ribosomal inhibition of BRCA is introduced, cells become less receptive to the agents.
BRCA has applications in predicting the phenotypic manifestations of breast cancer. It is important to note that BRCA tumors are basal-like in etiology. Studies have found a significantly higher number of basal-like tumors associated with germline mutations in BRCA1. Basal-like tumors tend to involve higher risks for patients; BRCA1 tumors implicated in this investigation were found to be HER2, ER, and PgR negative. BRCA1 tumors were also found to be more chemosensitive than BRCA2 tumors. In ovarian cancer, the susceptibility of BRCA cancer to chemotherapeutic agents was likewise observed. Specifically, breast cancer patients with BRCA mutations were best treated with neoadjuvant drugs like cisplatin and gemcitabine, while ovarian cancer patients benefitted from platinum-based chemotherapy. Thus, BRCA has significant biomarker properties defining its relevance in oncology. [20]
A study recently published by Danza, et al. documents the efficacy of microRNA molecules miR-573 and miR-578 as targets for BRCA mutations. These molecules interfere with the HIF-1 and VEGF pathways, preventing angiogenesis and the successful growth of BRCA tumors. This suggests that synthetic miR-573 and miR-578 could provide novel therapy options, as research on BRCA continues to develop. [21]
Ongoing Research and Conclusions
The aforementioned therapies remain under investigation. As these options become viable for many patients, clinical trials are underway to confirm their efficacy.
Specifically, trials with PARPi have become prominent. Drugs of interest include cediranib, an inhibitor of the VEGF receptor, and olaparib, a classic PARPi medication. Studies have demonstrated the efficacy of combination treatment with these drugs. [18]
As research continues on targeted therapy, more research is needed on specific strategies for combatting BRCA tumors. Additional targets should involve multiple stages of gene expression in order to optimize their success.
Acknowledgements
We thank Jocelyn Wong for generating the figures used in this paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. BRCA1 and BRCA2: Cancer risk and genetic testing fact sheet. Reviewed 01 April 2015 National Institutes of Health: National Cancer Institute. http://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet
2. U.S. National Library of Medicine: National Institutes of Health. Breast cancer. Updated 08 February 2017. https://www.nlm.nih.gov/medlineplus/breastcancer.html
3. Cancer of the breast (female). Revisited April 2016 National Institutes of Health: National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html
4. Cancer of the breast (female). Revisited April 2016 National Institutes of Health: National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html
5. Breast cancer types: what your type means. Revisited 06 February 2015 Rochester, Minnesota, United States: Mayo Clinic. http://www.mayoclinic.org/diseases-conditions/breast-cancer/in-depth/breast-cancer/art-20045654?pg=1
6. Hall JM, Lee MK, Newman B. et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684-1689
7. Wooster R, Bignell G, Lancaster J. et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789-792
8. Smith SC, Theodorescu D. Figure 1: Metastasis suppressor genes and steps in the metastatic cascade in human cancer. Nat Rev Cancer. 2009;9:253-264
9. Metastatic cancer. Updated 06 February 2017 National Institutes of Health: National Cancer Institute. http://www.cancer.gov/about-cancer/understanding/what-is-cancer/metastatic-fact-sheet#q3
10. Breast cancer treatment (PDQ)-patient version. Updated 12 October 2017 National Institutes of Health: National Cancer Institute. http://www.cancer.gov/types/breast/patient/breast-treatment-pdq
11. National Center for Biotechnology Information: U.S. National Library of Medicine. BRCA 1, DNA repair associated [Homo sapiens (human)]. Updated 20 February 2017. http://www.ncbi.nlm.nih.gov/gene/672
12. National Center for Biotechnology Information: U.S. National Library of Medicine. BRCA 2, DNA repair associated [Homo sapiens (human)]. Updated 20 February 2017. http://www.ncbi.nlm.nih.gov/gene/675
13. U.S. National Library of Medicine: National Institutes of Health. BRCA1 gene, Updated 28 February 2017. https://ghr.nlm.nih.gov/gene/BRCA1#sourcesforpage
14. Yan M, Rayoo M, Takano EA. et al. BRCA1 tumours correlate with a HIF-1α phenotype and have a poor prognosis through modulation of hydroxylase enzyme profile expression. Brit J Cancer. 2009;101:1168-1174 http://www.nature.com/bjc/journal/v101/n7/full/6605287a.html
15. Benafif S, Hall M. An update on PARP inhibitors for the treatment of cancer. OncoTargets and Therapy. 2015;8:519-528 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348139/
16. Tmor angiogenesis and the VEGF pathway. Genetech. https://www.biooncology.com/pathways/vegf/vegf-tumor-angiogenesis.html
17. Soponaro C, Malfettone A, Ranieri G. et al. VEGF, HIF-1a expression and MVD as an angiogenic network in familial breast cancer. PLoS ONE 8(1): e53070.
18. Benafif S, Hall M. An update on PARP inhibitors for the treatment of cancer. OncoTargets and Therapy. 2015;8:519-528 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348139/
19. Obermiller PS, Tait DL, Holt JT. Gene therapy for carcinoma of the breast: Therapeutic genetic correction strategies. Breast Cancer Res. 2000;2:28-31 https://breast-cancer-research.biomedcentral.com/articles/10.1186/bcr26
20. James CR, Quinn JE, Mullan PB. et al. BRCA1, a potential predictive biomarker in the treatment of breast cancer. The Oncologist. 2007;12(2):142-150 http://theoncologist.alphamedpress.org/content/12/2/142.full
21. Danza K, De Summa S, Pinto R. et al. MiR-578 and miR-573 as potential players in BRCA-related breast cancer angiogenesis. Oncotarget. 2015Jan;6(1):471-483
Author contact
![]() Corresponding author: Yi Lu, Ph.D., Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, Room 218, 19 South Manassas Street, Memphis, TN 38163 (USA) Tel.: (901) 448-5436 Fax.: (901) 448-5496 E-mail: yluedu
Corresponding author: Yi Lu, Ph.D., Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, Room 218, 19 South Manassas Street, Memphis, TN 38163 (USA) Tel.: (901) 448-5436 Fax.: (901) 448-5496 E-mail: yluedu