Oncomedicine 2017; 2:138-141. doi:10.7150/oncm.20759 This volume Cite
Review
First and Best Treatments for EGFR and PD-L1 - Competition for First Line Therapy in Adenocarcinoma
1. Pulmonary Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece;
2. Co-Director, Laboratory of Proteomics and Protein Sciences, Veterans Affair Health System, Baltimore, MD, USA;
3. Sana Clinic Group Franken, Department of Cardiology / Pulmonology / Intensive Care / Nephrology, “Hof” Clinics, University of Erlangen, Hof, Germany;
4. Johns Hopkins University School of Medicine, Baltimore, MD, USA;
5. Department of Respiratory & Critical Care Medicine, Changhai Hospital, the Second Military Medical University, China;
6. CarbioThoracic Department, “Theageneio” Anticancer Hospital, Thessaloniki, Greece;
7. Surgery Department, “Interbalkan” European Medical Center, Thessaloniki, Greece;
8. Pharmacology-Clinical Pharmacology, Department of Clinical, Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki;
9. Respiratory and Critical Care Institute, Cleveland Clinic Abu Dhabi, Al Maryah Island, Abu Dhabi - UAE;
10. Oncology Department, University General Hospital of Larissa, University of Thessali, Larissa, Greece;
11. Pulmonary Department, “Theageneio” Anticancer Hospital, Thessaloniki, Greece.
Received 2017-4-26; Accepted 2017-6-2; Published 2017-7-1
Abstract
Lung cancer is still diagnosed at advanced stages since there are no blood markers or efficient prevention programs. Moreover, lack of early disease symptoms do lead patients for early diagnosis. In the past ten years novel targeted and non-targeted therapies have entered the market for advanced stage disease patients. Epidermal growth factor receptor and anaplastic lymphoma kinase agents are considered an excellent choice as first line treatment for adenocarcinoma. However; in the past few months PD-L1 >50% patients can be treated with immunotherapy as first line treatment. Moreover; second generation tyrosine kinase inhibitors are already on the market in the case of disease relapse in EGFR and ALK positive patients. In the current mini review we will focus on current up-to-date data regarding the best choice between tkis and immunotherapy as first line treatment for adenocarcinoma.
Keywords: EGFR, ALK, PD-L1, Immunotherapy.
Introduction
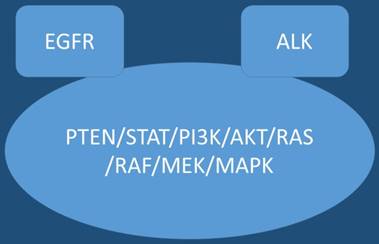
In the past ten years novel therapies such as tyrosine kinase inhibitors (TKIs) have entered the market for non-small cell lung cancer (nsclc) adenocarcinoma patients with epidermal growth factor receptor positive (EGFR) patients and anaplastic lymphoma kinase positive patients (ALK).[1-3] Upon disease relapse there are currently treatment options, for example in the case of EGFR patients erlotinib can be administered with bevacizumab.[4] In the case where mutation T790M is detected either by new biopsy in the site of relapse or by liquid biopsy osimertinib can be administered.[5, 6] The question whether to perform re-biopsy or liquid biopsy, tissue still remains the best material in order to investigate for mutation T790M, however; the choice between the two methods is mostly based on the performance status of the patient and center diagnostic tools.[6] Regarding ALK positive patients ceritinib is used after disease relapse.[7] Immunotherapy is currently also considered as first line treatment in the case of Programmed death-ligand 1 (PD-L1) >50% with the drug pembrolizumab.[8] Until now immunotherapy was only considered an option for second line treatment with nivolumab in nsclc patients.[9] Tyrosine kinase inhibitors have recently acquired licensed and can be used as second line treatment in non-positive EFGR patients in squamus cell carcinoma.[10] Moreover; novel non-specific agents are currently with efficiency such nab-paclitaxel in nsclc.[11] However; there is an issue that has to be addressed, which therapy is considered based on current data the best choice for first line treatment in the case of nsclc adenocarnoma patient positive for EGFR or ALK and PD-L1 >50% overexpression. Current data based on keynote 024 indicate that for these patients tyrosine kinase inhibitors should be initiated.[12-14] Figure 1.
EGFR; Epidermal Growth Factor, AKT; kinase-interacting protein1, PTEN; Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase, PI3K; Phosphatidylinositol-3-kinases, PI 3-kinases, PI(3)Ks, RAS; Ras superfamily of proteins, which are all related in 3D structure and regulate diverse cell behaviours, RAF; Raf kinases (more avidly C-Raf than B-Raf), MAPK/ERK; extracellular signal-regulated kinases , TKI; Tyrosine kinase inhibitor, STAT; Signal Transducers and Activators of Transcription. MEK; mitogen-activated protein kinase kinase enzymes MEK1 and/or MEK2.
Tyrosine Kinase Inhibitors
Epidermal growth factor positive patients can use three different drugs; erlotinib, gefitinib and afatinib.[15, 16] Unfortunately disease relapse occurs and treatment has to change. In the case of erlotinib the anti-vascular endothelial growth factor (VEGF) bevasizumab can be added in order to enhance the already administered treatment.[17] Gefitinib is the drug that has been mostly investigated along with the underlying molecular pathways that have been altered along the way of administration.[18-20] There are two treatment approaches, one could which to chemotherapy and the choice of investigating for the mutation T790M.[5, 21] The investigation of T790M can be done with re-biopsy in the site of disease relapse or with liquid biopsy.[6] Current data indicate that erlotinib plus bevasizumab can be administered to T790M positive patients with favorable results.[22] In another recently published study immunotherapy with nivolumab in EGFR positive patients, disease relapse and negative for T790M presented favorable data due to the high expression of PD-L1 levels. It has been observed that T790M positive patients have a lower PD-L1 expression.[23] Current data on tki combination and immunotherapy indicate that there is synergistic positive effect, however; increased toxicity has been observed.[24]
PD-L1
It has been observed that atezolizumab, pembrolizumab and nivolumab increase overall survival in second-line treatment of Stage III/IV squamous and non-squamous NSCLC when compared to taxane monotherapy. Keytruda increased progression-free survival in the first-line treatment of Stage IV NSCLC when >50% PD-L1 expression was observed when compared to platinum-based chemotherapy.[25] The toxicity observed was immune related as previously observed with similar checkpoint inhibitors. The use of pembrolizumab is based on the diagnostic test, Dako's IHC 22C3, to assess PD-L1 status of patients. Nowadays there are ongoing studies evaluating pembrolizumab in small-cell lung cancer and malignant pleural mesothelioma.[14] First data indicate that pembrolizumab appears to be well tolerated and could be used in patients with PD-L1-positive and malignant pleural mesothelioma. However; further investigation for response durability and efficacy evaluation in this patient population is needed.[26] It has been observed that PD-1/PD-L1 inhibitors are overall better tolerated than chemotherapy. In previously published studies PD1/PD-L1 inhibitors were associated with a lower risk of treatment-related symptoms (fatigue, nausea neutropenia, anemia), but a higher risk of immune-related adverse events (colitis, orogonitis). It has been observed that PD1/PD-L1 inhibitors are overall better tolerated than chemotherapy. [27] In lung cancer adenocarcinoma PD-L1+ cases convergence and cavitation were more frequently than did PD-L1- cases. In contrast, surrounding ground glass opacities and air bronchogram were observed less frequently in PD-L1+ cases than in PD-L1- cases. These observations will prove helpful in identifying PD-L1-expressing adenocarcinoma by CT before immunotherapy.[28] Combination of pembrolizumab, permetrexed and platinum analogs could be an effective and tolerable first-line treatment option for patients with advanced non-squamous NSCLC. This combination is being further explored in an ongoing international, randomized, double-blind, phase 3 study.[29]
Conclusions
In the last year, novel immunotherapeutic agents aimed at the PD-1/PD-L1 axis were introduced as lung cancer treatment. The two immunotherapeutic agents greatly influenced our approach to the treatment of lung cancer in first and second line. The limited toxicity profile and the ability to treat for prolonged periods, is an excellent addition in the arsenal of the oncologist. Immune checkpoint modulation produces durable responses and overall survival benefits with less toxicity compared to the adverse effects of conventional chemotherapy.[25] However, as PD-L1 staining does not sufficiently discriminate responders from non-responders for all checkpoint inhibitors, there still is a need for a better predictive biomarker. Moreover; the efficacy and toxicity of immune checkpoint inhibitors in the elderly is unclear because current available studies involve mainly a low number of elderly patients and I/O therapies are based on the immune status of a patient. Furthermore; ongoing clinical research focuses on the development of PD-1 and PD-L1 inhibitor monotherapy in neoadjuvant and adjuvant NSCLC, since there is a gup in our knowledge for these patients.[30]
There is also much interest in using these drugs in combination with other treatment modalities including cytotoxic chemotherapies in the first-line NSCLC, other immunotherapies such as cytotoxic T-lymphocyte-associated protein 4 antagonists, radiotherapy and molecularly targeted agents, including EGFR and ALK inhibitors. Concurrent treatment with radiotherapy in order to increase the effect of checkpoint inhibitors.[31] The toxicity profile of a drug is often a deciding factor in second-line setting for NSCLC.[32] It has been observed that in EGFR-mutant advanced non-small cell lung cancer, immune checkpoint inhibitors do not improve overall survival over that with docetaxel. Moreover; there are mechanisms of acquired resistance to first-line tyrosine kinase inhibitor therapy that should be elucidated in order to have a targeted therapy selection of second-line treatment for these patients.[33] Tyrosine kinase inhibitors generate rapid response rate, however; the duration of response is modest and therefore a possible combination with checkpoint inhibitors might prove beneficial in terms of extending the duration of response or block treatment resistance.[34, 35] To date data based on keynote 024 indicate that for these patients tyrosine kinase inhibitors should be initiated.[12-14] There is the cost effect issue where TKIs are cheaper and their efficiency is well established, however; resistance is induced after 1≥ year of administration. Therefore further studies on this group of patients will elucidate the best treatment for these patients.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zaric B, Stojsic V, Panjkovic M, Tegeltija D, Stepanov V, Kovacevic T. et al. Clinicopathological features and relation between anaplastic lymphoma kinase (ALK) mutation and histological subtype of lung adenocarcinoma in Eastern European Caucasian population. Journal of Cancer. 2016;7:2207-12 doi:10.7150/jca.16768
2. Papadopoulou E, Tsoulos N, Tsirigoti A, Apessos A, Agiannitopoulos K, Metaxa-Mariatou V. et al. Determination of EGFR and KRAS mutational status in Greek non-small-cell lung cancer patients. Oncology letters. 2015;10:2176-84 doi:10.3892/ol.2015.3600
3. Zaric B, Stojsic V, Kovacevic T, Sarcev T, Tepavac A, Jankovic R. et al. Clinical characteristics, tumor, node, metastasis status, and mutation rate in domain of epidermal growth factor receptor gene in serbian patients with lung adenocarcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:1406-10 doi:10.1097/JTO.0000000000000242
4. Otsuka K, Hata A, Takeshita J, Okuda C, Kaji R, Masago K. et al. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer chemotherapy and pharmacology. 2015;76:835-41 doi:10.1007/s00280-015-2867-8
5. Saad N, Poudel A, Basnet A, Gajra A. Epidermal growth factor receptor T790M mutation-positive metastatic non-small-cell lung cancer: focus on osimertinib (AZD9291). OncoTargets and therapy. 2017;10:1757-66 doi:10.2147/OTT.S100650
6. Zarogoulidis P, Gaga M, Huang H, Darwiche K, Rapti A, Hohenforst-Schmidt W. Tissue is the issue and tissue competition. Re-biopsy for mutation T790: where and why? Clinical and translational medicine. 2017;6:6. doi:10.1186/s40169-017-0135-8
7. Bendaly E, Dalal AA, Culver K, Galebach P, Bocharova I, Foster R. et al. Treatment Patterns and Early Outcomes of ALK-Positive Non-Small Cell Lung Cancer Patients Receiving Ceritinib: A Chart Review Study. Advances in therapy. 2017 doi:10.1007/s12325-017-0527-6
8. Giroux Leprieur E, Dumenil C, Julie C, Giraud V, Dumoulin J, Labrune S. et al. Immunotherapy revolutionises non-small-cell lung cancer therapy: Results, perspectives and new challenges. European journal of cancer. 2017;78:16-23 doi:10.1016/j.ejca.2016.12.041
9. Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Annals of oncology: official journal of the European Society for Medical Oncology. 2017 doi:10.1093/annonc/mdx151
10. Hohenforst-Schmidt W, Zarogoulidis P, Steinheimer M, Benhassen N, Sardeli C, Stalikas N. et al. Second-line afatinib administration in an elderly patient with squamous cell carcinoma. Therapeutics and clinical risk management. 2017;13:341-3 doi:10.2147/TCRM.S130816
11. Weiss J, Force RW, Pugmire BA, Peterson T, Faria C, Margunato-Debay S. et al. Comparative Effectiveness and Resource Usage in Patients Receiving First-line Taxane-based Chemotherapy for Stage IV Non-Small-cell Lung Cancer in a US Community Oncology Setting. Clinical lung cancer. 2016 doi:10.1016/j.cllc.2016.12.008
12. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2016;375:1823-33 doi:10.1056/NEJMoa1606774
13. Addeo R. A new frontier for targeted therapy in NSCLC: clinical efficacy of pembrolizumab in the inhibition of programmed cell death 1 (PD-1). Expert review of anticancer therapy. 2017;17:199-201 doi:10.1080/14737140.2017.1286986
14. Somasundaram A, Burns TF. Pembrolizumab in the treatment of metastatic non-small-cell lung cancer: patient selection and perspectives. Lung Cancer. 2017;8:1-11 doi:10.2147/LCTT.S105678
15. Domvri K, Zarogoulidis P, Darwiche K, Browning RF, Li Q, Turner JF. et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. Journal of Cancer. 2013;4:736-54 doi:10.7150/jca.7734
16. Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K. et al. Treatment of non-small cell lung cancer (NSCLC). Journal of thoracic disease. 2013;5(Suppl 4):S389-96 doi:10.3978/j.issn.2072-1439.2013.07.10
17. Rosell R, Dafni U, Felip E, Curioni-Fontecedro A, Gautschi O, Peters S. et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. The Lancet Respiratory medicine. 2017 doi:10.1016/S2213-2600(17)30129-7
18. Patel HM, Pawara R, Ansari A, Noolvi M, Surana S. Design and synthesis of quinazolinones as EGFR inhibitors to overcome EGFR resistance obstacle. Bioorganic & medicinal chemistry. 2017;25:2713-23 doi:10.1016/j.bmc.2017.03.039
19. Hu Y, Qin X, Yan D, Cao H, Zhou L, Fan F. et al. Genome-wide profiling of micro-RNA expression in gefitinib-resistant human lung adenocarcinoma using microarray for the identification of miR-149-5p modulation. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39:1010428317691659. doi:10.1177/1010428317691659
20. Wang H, Zhang M, Li P, Zhang G, Yan X, Ma Z. Retrospective Analysis of Different Treatment Schemes After Gefitinib Resistance in Advanced Non-small Cell Lung Cancer. Clinical therapeutics. 2017;39:610-9 doi:10.1016/j.clinthera.2017.01.027
21. Qiao L, Wang J, Long G, Jiang Y. Sequential treatment of tyrosine kinase inhibitor and platinum-based doublet chemotherapy on EGFR mutant non-small cell lung cancer: a meta-analysis of randomized controlled clinical trials. OncoTargets and therapy. 2017;10:1279-84 doi:10.2147/OTT.S128187
22. Mitsudomi T. Combined bevacizumab and erlotinib treatment in patients with lung cancer with the T790M resistance mutation. The Lancet Respiratory medicine. 2017 doi:10.1016/S2213-2600(17)30134-0
23. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K. et al. Tumor Immune Microenvironment and Nivolumab Efficacy in EGFR Mutation-Positive Non-Small Cell Lung Cancer Based on T790M Status after Disease Progression During EGFR-TKI Treatment. Annals of oncology: official journal of the European Society for Medical Oncology. 2017 doi:10.1093/annonc/mdx183
24. Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert opinion on drug safety. 2017;16:465-9 doi:10.1080/14740338.2017.1300656
25. Iafolla MAJ, Juergens RA. Update on Programmed Death-1 and Programmed Death-Ligand 1 Inhibition in the Treatment of Advanced or Metastatic Non-Small Cell Lung Cancer. Frontiers in oncology. 2017;7:67. doi:10.3389/fonc.2017.00067
26. Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B. et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. The Lancet Oncology. 2017 doi:10.1016/S1470-2045(17)30169-9
27. Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. The oncologist. 2017;22:470-9 doi:10.1634/theoncologist.2016-0419
28. Toyokawa G, Takada K, Okamoto T, Shimokawa M, Kozuma Y, Matsubara T. et al. Computed Tomography Features of Lung Adenocarcinomas With Programmed Death Ligand 1 Expression. Clinical lung cancer. 2017 doi:10.1016/j.cllc.2017.03.008
29. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17:1497-508 doi:10.1016/S1470-2045(16)30498-3
30. Sgambato A, Casaluce F, Gridelli C. The role of checkpoint inhibitors immunotherapy in advanced non-small cell lung cancer in the elderly. Expert opinion on biological therapy. 2017;17:565-71 doi:10.1080/14712598.2017.1294157
31. Kumar R, Collins D, Dolly S, McDonald F, O'Brien ME, Yap TA. Targeting the PD-1/PD-L1 axis in non-small cell lung cancer. Current problems in cancer. 2016 doi:10.1016/j.currproblcancer.2016.12.002
32. Rossi A, Maione P, Santabarbara G, Sacco PC, Casaluce F, Sgambato A. et al. The safety of second-line treatment options for non-small cell lung cancer. Expert opinion on drug safety. 2017;16:471-9 doi:10.1080/14740338.2017.1297795
33. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T. et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017;12:403-7 doi:10.1016/j.jtho.2016.10.007
34. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine. 2010;362:2380-8 doi:10.1056/NEJMoa0909530
35. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology. 2011;12:735-42 doi:10.1016/S1470-2045(11)70184-X
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom
Corresponding author: Paul Zarogoulidis, M.D, Ph. D, Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom